We aim to bring innovative treatment options to patients with the greatest unmet medical need and change the way that cancer is treated

MACOV PROJECT
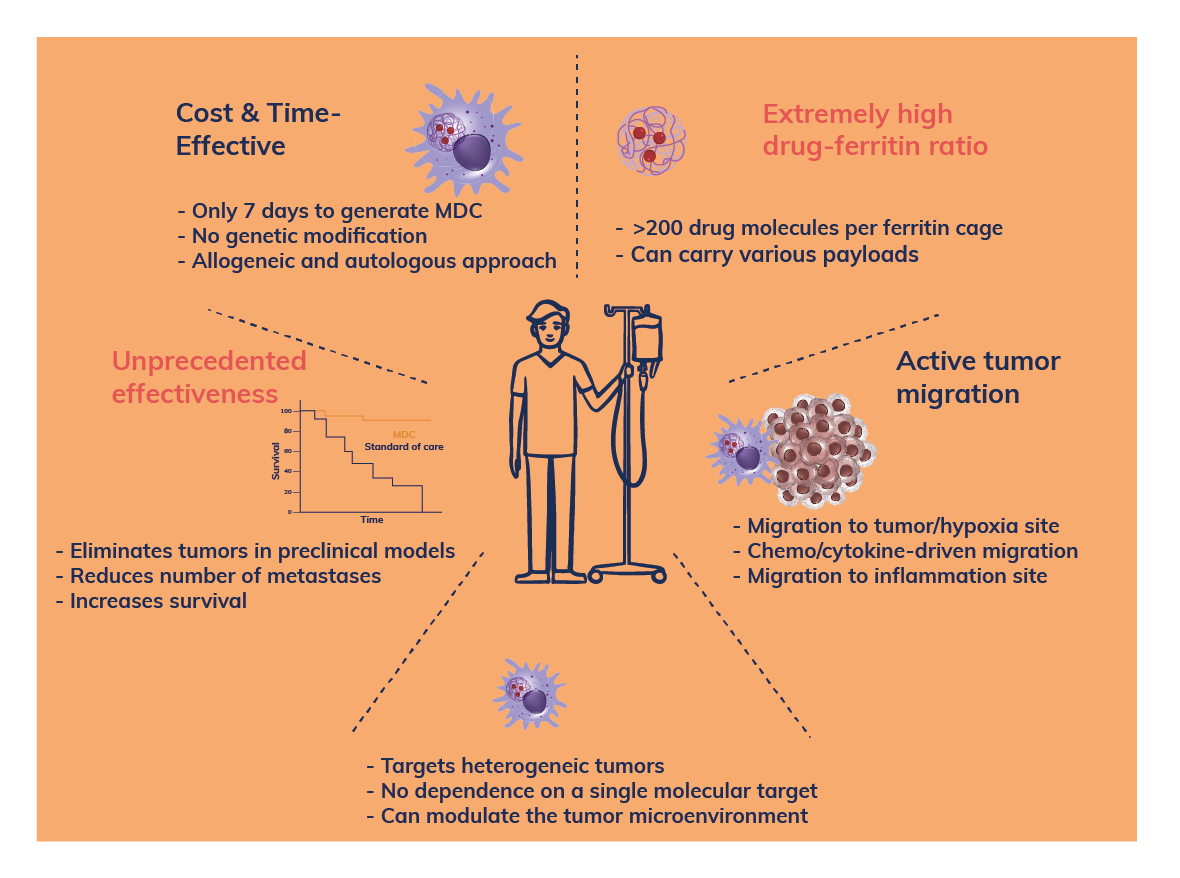
The project MACOV aims to develop an innovative allogeneic, off-the-shelf macrophage-based therapy for platinum-resistant ovarian cancer. This is a ground-breaking, first in class therapy that shows very high efficacy in multiple solid tumors of unmet medical need. It is based on a breakthrough discovery made by a team of scientists who subsequently founded the Cellis company.
In 2016, the European Research Council (ERC) awarded a “Starting Grant” to prof. Magdalena Król on the study of the molecular mechanism of the TRAnsfer of Iron-binding proteiN (TRAIN). In 2018, this project was selected as one of 10 interesting European projects in the last 10 years to be promoted during ERC 10th Anniversary. In 2020, the ERC Proof of Concept (PoC) grant “TROJAN” was awarded for further development of the project e.g. obtaining PoC in vivo using human macrophages and “humanized” cancer models. As a continuation, MACOV project is funded by the European Innovation Council (EIC) and the aim of this project is pre-clinical development of allogeneic MDC-735 for platinum-resistant ovarian cancer treatment.

OVARIAN CANCER
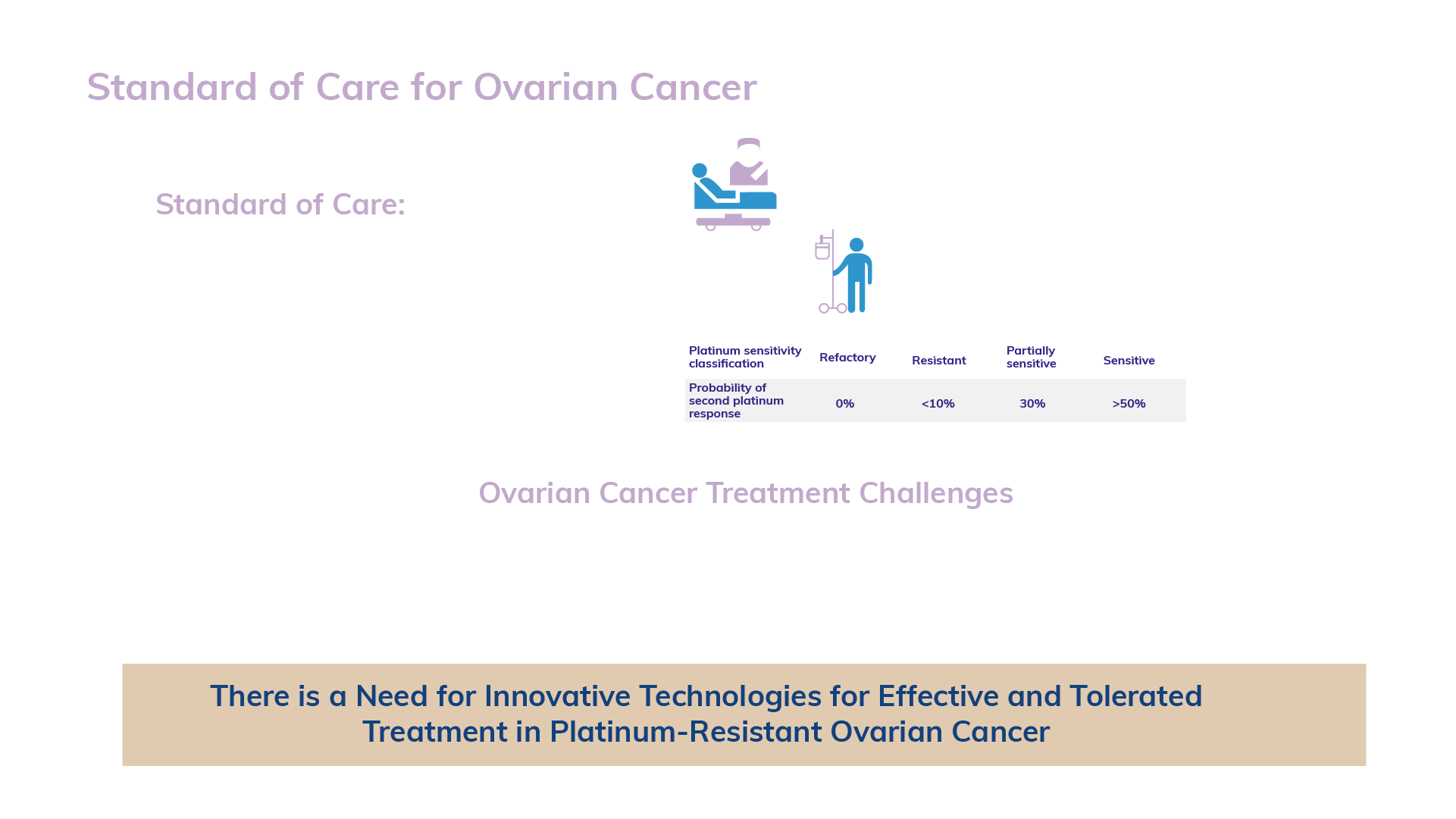
About 300,000 women are diagnosed with ovarian cancer each year and the number of deaths is estimated at 180,000 worldwide each year. Ovarian cancer metastasizes early in its development, often before it’s diagnosed. More than 60% of women with ovarian cancer are diagnosed with stage III or IV once it has spread beyond the ovaries. Ovarian cancer treatment involves surgery with frontline platinum-based chemotherapy. After relapse, patients receive second-line platinum-based chemotherapy and later, based on the outcome: PARP inhibitor or VEGF inhibitor maintenance treatment or active surveillance – chemotherapy or other treatments. As many as 15-30% of patients with ovarian cancer have primary platinum-resistant or refractory disease. After recurrence, generally 70% of advanced stage ovarian cancer relapses, and even in stage I or II patients, the relapse rate is 20-25%. The recurrence of ovarian cancer is now considered as a sign of an incurable disease (it becomes platinum-resistant). The average response rate to “salvage” therapy is only 10-15% with a median progression-free survival of 3-4 months.
CONTACT
e-mail: macov@cellis.eu
FUNDING
This project has received funding from the European Union’s Horizon 2022 research and innovation programme EIC Transition under grant agreement No. 101099232. Project started on November 1st, 2022.